Does fat from your plate displace fat from your thighs? Not necessarily
Recently there has been a push against the high fat in "high fat low carb", based on a combination of half-truths and outright contradictions. In this post, I'll address the simple and wrong idea that eating fat in particular on a low carb diet competes with fat loss.
Eating higher fat to get higher ketosis can't interfere with body fat use
One way that this has been expressed comes from Marty Kendall, in an ebook called "Big Fat Keto Lies", in which he sets up the following dilemma. Given that many consider the presence of ketone bodies as a way to track success, combined with the observation that many adapting to a ketogenic diet see progressively lower measurements of the ketone body β-hydroxybutyrate (BOHB) in the blood, one must either change the macronutrient composition of the diet in favour of fat, or give up the pursuit of higher BOHB. He asserts that the former would interfere with health and fat loss.
"This progressive adaptation leaves many people faced with the decision to either:
- continue to add more refined fat (e.g. butter, MCT oil, exogenous ketones etc.) to maintain elevated ketones to achieve ‘optimal ketosis’, or
- reduce dietary fat to allow fat from their body to be used, thus improving their metabolic health, reversing their diabetes and reducing or obliterating obesity."
I have discussed the phenomenon of lowered BOHB in the ketoadapted in detail in my previous post, Keto-adapted but no (low) ketones? Part II. We'll return to some relevant points brought up in this post later, specifically pertaining to the idea of "energy toxicity", but for now, let's just look at the dilemma. The idea is if you try to attain the levels of ketosis seen earlier in the keto-adaptation process, the fat that you are eating and using for energy is taking the place of energy that would otherwise have come from your fat stores.
The logical error here is plain when you think about what ketosis indicates.
As I've discussed in Eat meat. Not too Little. Mostly fat, ketosis requires fatty acid oxidation to be at maximum capacity in the liver. One bottleneck for fatty acid oxidation is simply the supply of fatty acids in the bloodstream. It is only when there is more fat than can be oxidised in the liver at a time that ketone bodies are made. That means that the presence of ketone bodies can be considered a marker for how much fat is being oxidised in the liver.With that in mind, consider the following.
Suppose you are keto-adapted. Let's compare a few different possibilities.
- You have eaten nothing but the minimum amount of protein to keep you in nitrogen balance. This is called a PSMF — Protein Sparing Modified Fast, and has a long history.
- In addition to (1) you have eaten fat to satiation.
- In addition to (1) you have eaten protein to satiation.
The important fact is this: if your BOHB goes up when you add fat to a diet of adequate protein, as in (2), that means that you burned more fat in addition to the maximum amount of fat your adipose tissue was able to release on a PSMF.
Think about that for a minute. The fat that you ate that caused a rise in BOHB could not have replaced fat that would otherwise have come from your fat stores. If it did, then BOHB wouldn't have gone up! What it did was give you more energy derived from fat than you would otherwise have had access to. It does not necessarily mean that you got more fat from your body (though see below for why that's not impossible), but it certainly does not mean that you got less.
Is it possible that you overshot, and are contributing to both energy use and storage? Yes, of course. Many have recently called into question overgeneral assumptions about the causal role of insulin levels in obesity. Nonetheless, whether you are predominantly storing or burning fat is indeed largely a function of insulin, as described in lay terms by [Phi2020]. One clue as to whether you have eaten so much fat that it's contributing not just to "energy out" , as it were, but also storage, might be if your BOHB is levelling off, or if you are gaining fat.
However, the basic argument, that increasing fat intake in order to increase BOHB must displace adipose fat for oxidation, doesn't hold up to scrutiny because the very fact that BOHB is going up means more fat is being oxidised than was without the higher fat.In other words, if reducing fat intake lowers BOHB, then your body fat is not stepping up to be used, whatever the reason.The lower BOHB demonstrates this.
As such, the dilemma in its basic form fails.
Lower ketosis from higher protein reflects either lower lipolysis or lower hepatic fat oxidation
What about scenario (3), where you eat more protein than you "needed"? (Note that we aren't individually measuring nitrogen balance, just guessing.) If your BOHB stays the same when you add more protein to a PSMF, then you apparently haven't interfered with fat loss by eating more protein. That's great.
If BOHB actually goes down, there are two possibilities. It could mean less fat is available in the bloodstream to be oxidised. This would imply an effect of the protein on fat release, perhaps via insulin. Or it could mean that fat oxidation has been reduced with the same supply. What reduces fat oxidation? A major determinant of how much fat can be oxidised, given a certain amount in the blood, is glucose availability [Wol1998]. So another reason to suspect if your BOHB goes down with higher protein intake is that you've introduced more glucose. Given that protein raises blood glucose in the ketogenic state, this seems likely to be at least a contributor. If this is the case — if you've got the same circulating fat, but less oxidation, that fat will be re-esterified into triglycerides, instead of transformed into ketone bodies.
In other words, if BOHB goes down in scenario (3), that's an indication that fat is being oxidised at a lower rate. If less fat is being oxidised, the either you are using less energy, or you are making up for it with another fuel source. Since the fuel source you add was protein, that would mean metabolising the additional protein for energy, which is not catastrophic, by any means. But you must understand that insofar as you are deriving energy from that protein, it would be in fact, replacing energy from fat from your adipose tissue. Protein from your plate would be displacing fat from your thighs. Regardless, in that scenario, the higher protein is either reducing the release of fat from your fat stores, or, if it didn't do that, then it is transforming more of the fat that is in circulation into triglycerides.
Lower ketosis at the same circulating fat level means more triglycerides
Let's think about this in the context of the "Lean Mass Hyper-Responder", LMHR, as discussed in the article above discussing low BOHB. LMHR describes those with a common response to a low carb diet, particularly (but not exclusively) lean, athletic people. It is characterised by a fasting (overnight fasted) lipid profile of low triglycerides, high HDL, and high LDL. Although not part of the criteria, LMHRs also often report moderately elevated fasting glucose. What we are looking at is a transformation of the energy form. The high LDL comes from having had many hours of higher triglycerides travelling in VLDL that leave triglyceride-poor particles (LDL) behind when depleted by the overnight fast. This would seem to reflect a tradeoff from having high energy in the form of free fatty acids and ketone bodies to having higher glucose and triglyceride-rich lipoproteins. If you are going to make an argument about energy in the blood being toxic, then you have to count this evidence from glucose, and from LDL and other triglyceride-rich lipoproteins as well. This tradeoff is what the Feldman Drop Protocol demonstrates, which motivates the "Energy Model" — the observation that lipoproteins have far more roles than carrying cholesterol. The results of this protocol are also consistent with reports from Paleomedicina, a clinic that uses a high fat, low protein, animal foods diet therapeutically. They observe that compared to a higher protein "carnivore" diet, higher fat and lower protein "normalises" blood glucose and LDL cholesterol.
"But I tried higher fat and I gained fat"
When considering the effect of "added" fat, the nuance is the baseline PSMF. If you start with a level of protein that is a lot higher than adequate, adding more fat may be the worst of both worlds, because when protein gets sufficiently high it will begin to interfere with the hormonal signals that determine whether fat is primarily stored or primarily released. Protein intake increases both insulin and glucagon. As eloquently described by Ben Bikman, the higher the ratio of the two, the more proportionally that rise will be in insulin. So a small initial difference in insulin can magnify the insulin response to protein, changing it from essentially neutral in insulin-to-glucagon terms, to an ever-increasing positive feedback loop. In other words, if your basal insulin is higher than ideal, this effect will kick in sooner.
Protein either raises the insulin-to-glucagon ratio or it raises blood sugar
On the other hand, suppose you have a low insulin-to glucagon ratio, such that protein doesn't disturb it. As mentioned above, blood glucose will then rise. This is normal in the ketogenic condition.
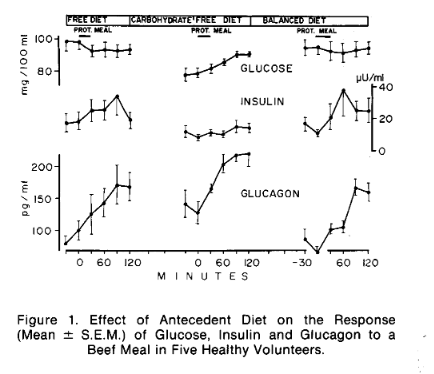
(Image from [Mul1971].)
With the higher glucose situation created by increased protein you would, as described above, limit fat oxidation (and ketogenesis) and increase triglyceride synthesis.
A metabolically healthy bodybuilder or endurance athlete with low insulin may be able to consume more protein for energy while still getting easy access to his own fat than someone with higher basal insulin or estrogen. This may be the difference between those who can thrive on higher protein and lower fat intake and those who can't. But even the metabolically healthy should expect to limit fat oxidation with higher protein. So high fat and high protein together could be counter-productive.
Rabbit starvation
If you're in the category of people for whom higher protein reduces fat release, low fat intake will simply result in low energy. In fact, it would look a lot like rabbit starvation, a phenomenon described by explorers in which illness develops from a diet of only lean meat. Speth and Spielman have collected many descriptions of this [Spe1983]. Here are just a couple:
If you are transferred suddenly from a diet normal in fat to one consisting wholly of rabbit you eat bigger and bigger meals for the first few days until at the end of about a week you are eating in pounds three or four times as much as you were at the beginning of the week. By that time you are showing both signs of starvation and of protein poisoning. You eat numerous meals; you feel hungry at the end of each; you are in discomfort through distention of the stomach with much food and you begin to feel a vague restlessness. Diarrhoea will start in from a week to 10 days and will not be relieved unless you secure fat. Death will result after several weeks. (Stefansson 1944:234)
...
If people had only rabbits at such times they would probably starve to death, because these animals are too lean. The same might be true if they could get only thin moose. People cannot live on lean meat alone, but if they have enough fat they can survive indefinitely. (Kutchin, Alaska; Nelson 1973: 142)
...
We tried the meat of horse, colt, and mules, all of which were in a starved condition, and of course not very tender, juicy, or nutritious. We consumed the enormous amount of from five to six pounds of this meat per man daily, but continued to grow weak and thin, until, at the expiration of twelve days, we were able to perform but little labor, and were continually craving for fat meat. (Marcy I863:16)
Some have proposed that rabbit starvation could only happen in lean people. But this ignores a basic common finding in obesity that fat is less readily released or used. If you have this problem, which could be why you are fat in the first place, then for energy purposes it's as if you had less fat mass.
We don't want low energy! We want high available energy to feel good and for our bodies to do what they need to do to function best. This is not toxicity, it is vitality.
Could I actually access more body fat with higher fat intake?
On the other hand, consuming primarily fat, and relatively lower protein, should maximise the chance of using fat for energy, by lowering glucose, a primary inhibitor of fat oxidation, as much as possible. If you have a relatively high baseline insulin to glucagon ratio, lowering protein should also help increase lipolysis by minimising the insulin response. A happy coincidence is that when you burn fat at a higher rate, it also leaves more glycerol, the byproduct of breaking up triglycerides. Glycerol is a substrate for gluconeogenesis; thus burning more fat spares protein [1]. Likewise, higher ketosis makes more acetone available, which is also used for gluconeogenesis, sparing protein [2]. In other words, increased reliance on fat for energy can reduce the need for protein for GNG by providing alternative substrates.
So it's actually reasonable to predict that by lowering protein you could burn more fat in total, while also using some of its byproducts to make up for some of the protein. If so, then given the same total calories with less protein and more fat, you could conceivably burn more body fat than if protein were higher and fat lower, due to higher lipolysis and higher fat oxidation rates. This would have to entail more energy expenditure, of course, (calories in, calories out can't be denied), which could go into body repair, synthesis of proteins or hormones, or just feeling the urge to move more.
In Sum
Not only is it contradictory to predict that higher fat intake leading to higher ketosis must reduce the use of fat from fat stores, it could, in fact increase it.
Of course, type of fat, and what comes with it also matter! If you saw weight gain with "high fat keto" based on nuts and cream cheese, you would not be alone. Placing the blame on the fat is like calling the result of a Happy Meal the fault of the beef.
References
| [Mul1971] Muller, Walter A., Gerald R. Faloona, and Roger H. Unger. “The Influence of the Antecedent Diet upon Glucagon and Insulin Secretion.” New England Journal of Medicine 285, no. 26 (December 23, 1971): 1450–54. https://doi.org/10.1056/NEJM197112232852603. |
| [Phi2020]
Virta Health. “Is Dietary Fat Burned before Stored Fat on a Ketogenic Diet?,” February 4, 2020. https://virtahealth.webflow.io/faq/dietary-fat-burned-before-stored-fat-keto. "For regular dietary fats, once they are digested, they enter the circulation and participate in what is called ‘fatty acid turnover.’ Whether fed or fasted, the body is always releasing, burning, and storing fat. When insulin is high, storage predominates, but turnover continues. When insulin is low, release and oxidation predominate. If you eat fat along with a lot of carbohydrates, it is prone to be stored. When fat is consumed in the context of a well formulated ketogenic diet, it—along with fat released from adipose stores—is prone to be burned. But once digested and absorbed, dietary fat and stored fat enter the ‘turnover pool’ and are in a constant state of mixing." |
|
[Pic2018]
Piché, Marie-Eve, Siôn A Parry, Fredrik Karpe, and Leanne Hodson. “Chylomicron-Derived Fatty Acid Spillover in Adipose Tissue: A Signature of Metabolic Health?” The Journal of Clinical Endocrinology & Metabolism 103, no. 1 (January 1, 2018): 25–34. https://doi.org/10.1210/jc.2017-01517. "There is a considerable degree of spillover FA into the systemic NEFA pool in the postprandial state; this process is greater and more dynamic in lean individuals and women. Contrary to general perception, spillover of chylomicron-derived FA into systemic circulation is a physiologically normal feature most easily observed in people with a higher capacity for clearance of plasma triglycerides, but does not appear to be a pathway providing excess NEFA in obesity." |
| [Spe1983] Speth, John D, and Katherine A Spielmann. “Energy Source, Protein Metabolism, and Hunter-Gatherer Subsistence Strategies.” Journal of Anthropological Archaeology 2, no. 1 (March 1983): 1–31. https://doi.org/10.1016/0278-4165(83)90006-5. |
| [Wol1998]
Wolfe, R R. “Metabolic Interactions between Glucose and Fatty Acids in Humans.” The American Journal of Clinical Nutrition 67, no. 3 (March 1, 1998): 519S-526S. https://doi.org/10.1093/ajcn/67.3.519S. "[T]he availability of plasma fatty acids is not a primary determinant of the rate of fat oxidation. Rather, different rates of fat oxidation for a particular [rate of appearance] are determined by the availability of glucose. As a consequence, unmetabolized fatty acids are cleared from the blood and reesterified into triacylglycerol. The liver is a major site of fatty acid clearance. Those fatty acids taken up by the liver that are not oxidized are reesterified into triacylglycerol and secreted into the blood as VLDL triacylglycerol. In general, the rate of VLDL triacylglycerol secretion is related to the availability of fatty acids (31). However, during periods of hyperglycemia and hyperinsulinemia, hepatic oxidation of fatty acids is low, and fatty acids that are taken up by the liver are more efficiently channeled into triacylglycerol." |
| [1]
See eg. H, Baba, Zhang Xj, and Wolfe Rr. “Glycerol Gluconeogenesis in Fasting Humans.” Nutrition (Burbank, Los Angeles County, Calif.) 11, no. 2 (March 1, 1995): 149–53. Landau, B. R., J. Wahren, S. F. Previs, K. Ekberg, V. Chandramouli, and H. Brunengraber. “Glycerol Production and Utilization in Humans: Sites and Quantitation.” The American Journal of Physiology 271, no. 6 Pt 1 (December 1996): E1110-1117. https://doi.org/10.1152/ajpendo.1996.271.6.E1110. Wang, Yujue, Hyokjoon Kwon, Xiaoyang Su, and Fredric E. Wondisford. “Glycerol Not Lactate Is the Major Net Carbon Source for Gluconeogenesis in Mice during Both Short and Prolonged Fasting.” Molecular Metabolism 31 (November 9, 2019): 36–44. https://doi.org/10.1016/j.molmet.2019.11.005. Shah, Ankit M., and Fredric E. Wondisford. “Tracking the Carbons Supplying Gluconeogenesis.” The Journal of Biological Chemistry 295, no. 42 (October 16, 2020): 14419–29. https://doi.org/10.1074/jbc.REV120.012758. |
| [2] See previous post https://www.mostly-fat.com/mostly-fat/2020/10/keto-adapted-but-no-low-ketones-part-ii/#rei1979 |
